
These corpuscles would later be named “electrons”, based on the theoretical particle predicted by Anglo-Irish physicist George Johnstone Stoney in 1874. To explain the overall charge of the atom, which consisted of both positive and negative charges, Thompson proposed a model whereby the negatively charged “corpuscles” were distributed in a uniform sea of positive charge – known as the Plum Pudding Model.
This effectively disproved the notion that the hydrogen atom was the smallest unit of matter, and Thompson went further to suggest that atoms were divisible. The Plum Pudding model of the atom proposed by John Dalton. He concluded that rather than being composed of light, they were made up of negatively charged particles that were 1ooo times smaller and 1800 times lighter than hydrogen.
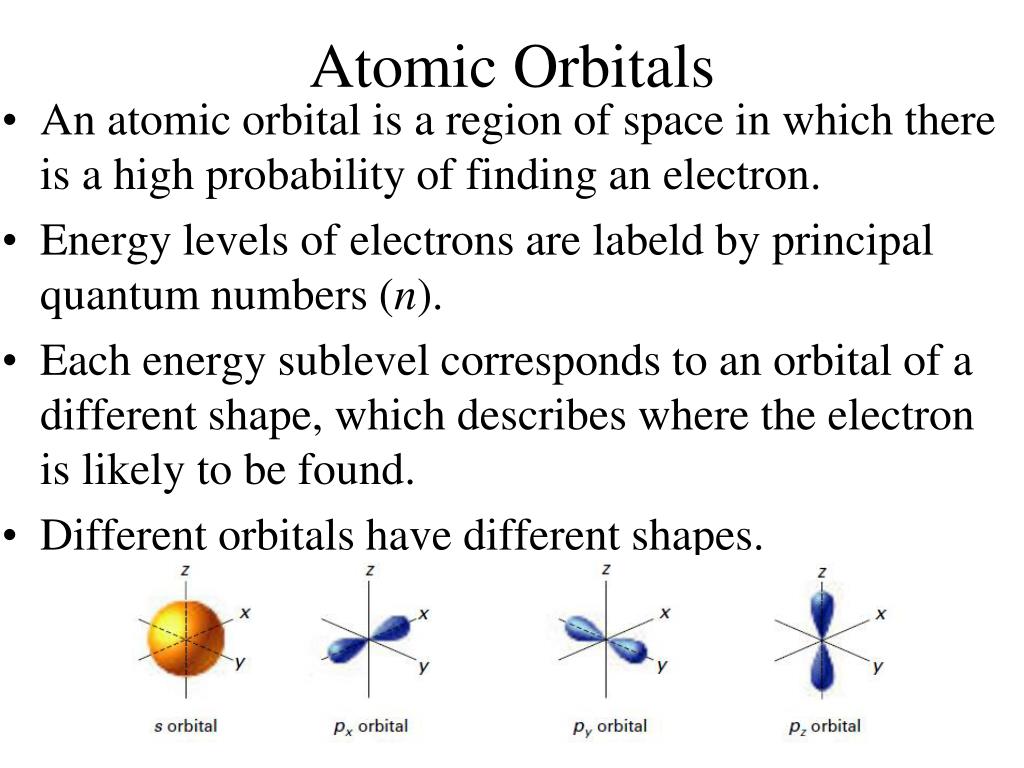
#Electron cloud model and atomic orbitals series#
Through a series of experiments using cathode ray tubes (known as the Crookes’ Tube), Thomson observed that cathode rays could be deflected by electric and magnetic fields. By the end of the 19th century, his would change drastically, thanks to research conducted by scientists like Sir Joseph John Thomson. However, most scientists ventured that this unit would be the size of the smallest known atom – hydrogen. Discovery Of The Electron:īy the late 19th century, scientists also began to theorize that the atom was made up of more than one fundamental unit. This theory expanded on the laws of conversation of mass and definite proportions and came down to five premises: elements, in their purest state, consist of particles called atoms atoms of a specific element are all the same, down to the very last atom atoms of different elements can be told apart by their atomic weights atoms of elements unite to form chemical compounds atoms can neither be created or destroyed in chemical reaction, only the grouping ever changes. Through a series of experiments involving gases, Dalton went on to develop what is known as Dalton’s Atomic Theory.

For example, in the early 1800’s, English scientist John Dalton used the concept of the atom to explain why chemical elements reacted in certain observable and predictable ways. It was not until the 19th century that the theory of atoms became articulated as a scientific matter, with the first evidence-based experiments being conducted.

Various atoms and molecules as depicted in John Dalton’s A New System of Chemical Philosophy (1808). However, this theory was more of a philosophical concept than a scientific one. The term “atom” was coined in ancient Greece and gave rise to the school of thought known as “atomism”. The earliest known examples of atomic theory come from ancient Greece and India, where philosophers such as Democritus postulated that all matter was composed of tiny, indivisible and indestructible units. Hence, their locations could only be described as being part of a ‘cloud’ around the nucleus where the electrons are likely to be found.

Instead, Schrodinger proposed a model whereby scientists could only make educated guesses as to the positions of electrons. Thanks to this model, electrons were no longer depicted as particles moving around a central nucleus in a fixed orbit. One such example is the Electron Cloud Model proposed by Erwin Schrodinger. Thanks to ongoing studies on the behavior of electrons, scientists began to propose theories whereby these elementary particles behaved in ways that defied classical, Newtonian physics. In addition to Ernest Rutherford and Niels Bohr giving birth to the Standard Model of particle physics, it was also a period of breakthroughs in the field of quantum mechanics. The early 20th century was a very auspicious time for the sciences.


 0 kommentar(er)
0 kommentar(er)
